Agricultural Literacy Curriculum Matrix
Lesson Plan
Plant Nutrient Deficiencies (Grades 9-12)
Grade Level
9 - 12
Purpose
Students will recognize that plants, like people, require essential nutrients to be present in the right amounts in order to be healthy, use reference materials to diagnose plant nutrient deficiencies, define fertilizer as a type of “food” for plants, and appreciate that fertilizers are used to replenish nutrients in agricultural soils. Grades 9-12
Estimated Time
1 hour
Materials Needed
Activity 1: When a Plant Needs "Food"
- No materials needed
Activity 2: Humanity Against Hunger
- Different colored pens or pencils
- Lesson Handouts:
- If completing print-based activity:
- Master 4.1, “Humanity Against Hunger” (to project)
- Masters 4.2a–c, Corn Case Studies (1 per team of 2–3 students)
- Master 4.3, Plant Doctor Evaluation Form (1 per team of 2–3 students)
- Masters 4.4a–d, Plant Doctor Reference Manual (1 per team of 2–3 students)
- Master 4.5, Crops, Soil, and Nutrients (1 per student and 1 to project)
- If completing web-based activity:
- Master 4.3, Plant Doctor Evaluation Form (optional)
- Master 4.5, Crops, Soil, and Nutrients (1 per student and 1 to project)
- Case Study Answers
- If completing print-based activity:
Optional Extension:
- Master 4.6, Investigating Calcium Deficiencies (1 per student and 1 to project)
- Master 4.7, Additional Experimental Results (1 to project)
- Master 4.8, Calcium and Plant Growth (1 per student)
- Master 4.9, EDTA Effects on Pea Plants (1 to project, optional)
- For the teacher
- Ethylenediaminetetraacetic acid, disodium salt, dehydrate (EDTA), Water, Sodium hydroxide (1 N), pH meter or pH test strips
- For each team of 3 students
- 6 paper or plastic cups*, Dissecting needle (or other tool for poking holes in bottom of cups), Potting soil, Tap water, EDTA solution (5 mM and 25 mM; after seeds have germinated), 6 pea seeds, Permanent marker pen, Ruler (after seeds have germinated), Stick or skewer (optional; may be helpful after plants have germinated to hold them upright)
* The size of the cup is not critical. Small paper cups (3-oz. bathroom size) are suitable for this activity. The size is adequate for growing the peas for the short duration of the investigation and requires less space and soil than larger cups.
Vocabulary
commercial fertilizer: commercially prepared mixtures with precisely measured plant nutrients that include nitrogen, phosphorus, and potassium applied to the soil to restore fertility and increase crop yields
inorganic fertilizer: commercially prepared mixtures of plant nutrients containing nitrogen, phosphorus, and potassium that was obtained by mining from the earth
macronutrient: a nutrient that must be present in a relatively large amount to ensure the health of the organism; building blocks used to make essential biomolecules
micronutrient: a nutrient required in small quantities to ensure the health of the organism; often used as cofactors for enzymatic reactions
nutrient: a substance that provides nourishment essential for growth and the maintenance of life
organic fertilizer: a fertilizer that undergoes little or no processing and includes plant, animal, and/or mineral materials
Background Agricultural Connections
Nutrient Deficiencies of Plants
People and plants are very different types of organisms. For example, people have blood, while plants have sap. People are consumers, while plants are producers. Despite their many differences, both people and plants are made up of cells. In order for cells to be healthy, they must have certain nutrients. If a person is lacking a needed vitamin, mineral, or essential element, then a deficiency is the result. We are familiar with the results of some nutrient deficiencies. For example, if a person lacks iron, he or she becomes anemic, or if a person lacks calcium, their bones become brittle. As discussed in Section 2.0, Plants and Their Essential Elements, plants require a variety of elements to be present in different amounts in order to support healthy growth. A nutrient deficiency results if a particular nutrient is not available in sufficient quantity to meet the needs of the growing plant. Nutrient toxicity occurs when a nutrient is present in such an excess that it harms the plant. Table 11 lists most of the essential plant nutrients and describes what happens when plants have too little or too much of them. 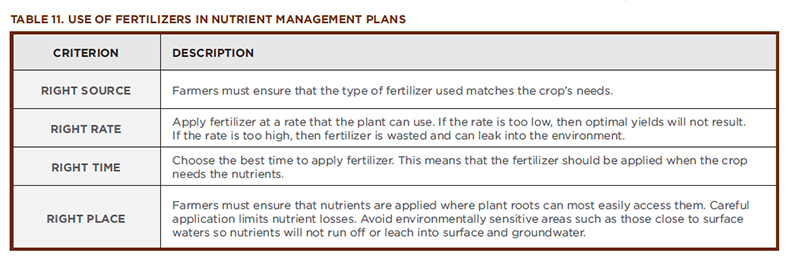
As shown in Table 11, when a plant is out of nutrient balance, it displays symptoms that are characteristic for that particular nutrient. A farmer concerned for the health of their crops must use scientific tools to prevent deficiencies and, if necessary, to examine these symptoms and diagnose problems, much like a physician does when encountering a patient with a dietary deficiency. Soil and plant tissue tests are used to detect nutrient imbalances. Once the problem has been identified, steps are taken to correct the imbalance. Farmers prescribe fertilizers for their crops in a manner similar to doctors prescribing vitamins for their patients. 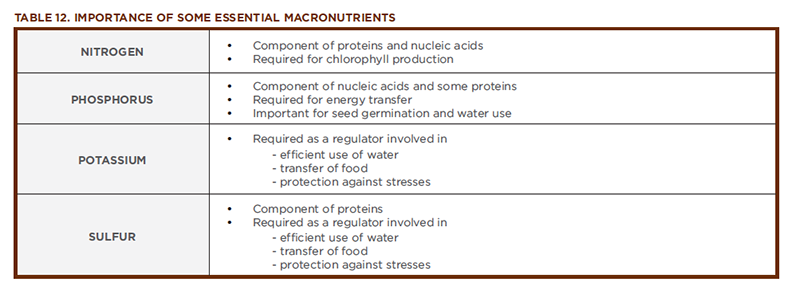
 Humans have been raising crops for nearly 10,000 years. Even ancient farmers fertilized their crops. The use of human and animal waste to increase soil fertility was recorded in China over 2,000 years ago. During the “golden age” of Greece from 800 to 200 BC, historians discussed methods for using sewage and classifying manures according to their value for crop production. Although these ancient cultures lacked our understanding of chemistry, they were observant and learned through trial and error how to help their crops grow. Mineral fertilizer in the form of saltpeter or potassium nitrate is mentioned by early Greek and Roman writers and in the Bible. Ancient Greeks also used salt brines to fertilize palm trees.
Humans have been raising crops for nearly 10,000 years. Even ancient farmers fertilized their crops. The use of human and animal waste to increase soil fertility was recorded in China over 2,000 years ago. During the “golden age” of Greece from 800 to 200 BC, historians discussed methods for using sewage and classifying manures according to their value for crop production. Although these ancient cultures lacked our understanding of chemistry, they were observant and learned through trial and error how to help their crops grow. Mineral fertilizer in the form of saltpeter or potassium nitrate is mentioned by early Greek and Roman writers and in the Bible. Ancient Greeks also used salt brines to fertilize palm trees.
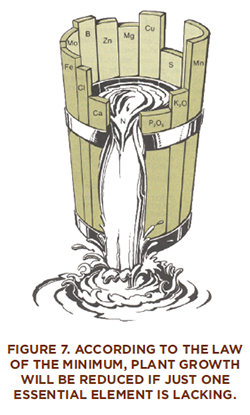
Justus von Liebig (1803–1873) is known as the founder of the modern fertilizer industry. Using the contributions of other scientists and his own discoveries, Liebig formulated the “mineral theory,” which held that crops “grow or diminish in exact proportion” to the amount of nutrient applied. Leibig stressed the value of replacing nutrients to maintain soil fertility. He also developed the “law of the minimum,” which states that if one essential element is deficient, then plant growth will be lacking even when all other essential elements are abundant. If the deficient element is supplied, then growth will increase up to the point where the supply is no longer the limiting factor.
The concept of the law of the minimum has been modified through the years as scientists have achieved a better understanding of the variables affecting plant growth. Moisture, temperature, insect control, weed control, light, plant population, and genetic capabilities of plant varieties are now part of this rule.
Today, commercial fertilizers are obtained from a variety of natural sources. The world’s first commercial fertilizer was sodium nitrate mined from natural deposits in Chile and imported into Europe and the United States starting around 1830. Around the same time, ammonium sulfate, a by-product of the manufacture of coal gas used for illumination, was sold as a commercial fertilizer.
Nitrogen (The Builder)
Nitrogen (N) is a primary building block for all organisms. It is a component of every amino acid and therefore essential to making proteins. As part of the chlorophyll molecule, nitrogen helps keep plants green. Nitrogen, along with magnesium, is the only element in the chlorophyll molecule that the plant obtains from the soil.
Vigorous plant growth is associated with adequate nitrogen nutrition, in part because nitrogen plays a key role in cell division. If cell division is slowed or stopped, so is leaf growth, which affects the surface area of the leaf exposed to the sun. A smaller surface area reduces the plant’s ability to produce biomass (yield). In addition to increasing yield, nitrogen also improves crop quality by increasing its protein content. Crop plants generally require more nitrogen to grow at their full potential than non-crop plants.
In 1918, scientists Fritz Haber (1868–1934) and Carl Bosch (1874–1940) were awarded the Nobel Prize for developing nitrogen fertilizer by synthesizing ammonia from nitrogen gas and hydrogen. While this process has been modified several times, today the Haber-Bosch process remains the method by which nitrogen fertilizer is commercially produced. Some academics have even suggested that this process has been of greater fundamental importance to the modern world than the invention of the airplane, nuclear energy, space flight, or television.22 The Haber-Bosch process has increased the amount of plant-available nitrogen produced on land by 60–70 percent compared to the natural processes of biological nitrogen fixation and lightning.26 Ammonia can be used in a wide variety of field conditions and is a major source of nitrogen applied to crops in the United States. Ammonia contains 82 percent nitrogen and is an important component for most nitrogen-based fertilizers. Another nitrogen source is urea, which is made by reacting ammonia with carbon dioxide and is  45 percent nitrogen.
45 percent nitrogen.
Organic sources of nitrogen have long been used as fertilizers. Between the years 1850 and 1900, the major natural organics were human excrement, cottonseed meals, fish scrap, and slaughterhouse wastes. In 1910, approximately 90 percent of the nitrogen used in the United States came from organic sources. As competition from commercial fertilizers grew, the contributions from organics decreased to 34 percent in 1920 and to 3.4 percent in 1950.
A new form of fertilizer was developed in the 1950s called activated sewage sludge. This material is made by passing wastewater through filters and centrifuging it to remove debris, oil, grease, and grit. The wastewater is then oxygenated to help microorganisms break down the biomass. Excess water is removed, and the final product is a thick, fibrous cake that is dried in kilns at high temperature to kill any remaining microorganisms or pathogens.
Phosphorus (The Energy Supplier)
Phosphorus (P) is found in every living cell. In plants, it serves as both a structural element and as a catalyst for biochemical reactions. Phosphorus is a component of DNA and ATP (the cell’s energy molecule). It also plays vital roles in capturing light during photosynthesis, helping with seed germination, and helping plants use water efficiently. Plants also use phosphorus to help fight external stress and prevent disease.
Animal and human bones contain insoluble calcium phosphate. As early as 2,000 years ago, Chinese farmers treated bones with lime and spread them on their fields. The lime treatment was necessary to convert the calcium phosphate into a more soluble form that plant roots could absorb. In the 1800s, fertilizer manufacturers wanted to produce phosphorus fertilizers that were more effective and plentiful than bones. They turned to natural deposits of phosphate rock in the fossilized remains of ancient marine life found in rock deposits around the world. The phosphate in these deposits exists in various forms of a very stable compound called apatite. To make the fertilizer called superphosphate, the phosphate rock is treated with acid or heat to render the phosphorus more soluble. Superphosphate production began in the United States in South Carolina in 1849.
Potassium (The Regulator)
 Potassium (K) is essential to the workings of every living cell. Although potassium is not a part of any important plant structure, it plays critical roles in several physiological processes. Potassium activates enzymes that catalyze chemical reactions involved with growth. It plays an important role in water balance by regulating the opening and closing of stomates (the pores in leaves through which gases are exchanged). Potassium also helps regulate the rate of photosynthesis through its role in the production of ATP. Other aspects of plant health influenced by potassium include the growth of strong stalks, protection from extreme temperatures, and the ability to fight stress and pests such as weeds and insects.
Potassium (K) is essential to the workings of every living cell. Although potassium is not a part of any important plant structure, it plays critical roles in several physiological processes. Potassium activates enzymes that catalyze chemical reactions involved with growth. It plays an important role in water balance by regulating the opening and closing of stomates (the pores in leaves through which gases are exchanged). Potassium also helps regulate the rate of photosynthesis through its role in the production of ATP. Other aspects of plant health influenced by potassium include the growth of strong stalks, protection from extreme temperatures, and the ability to fight stress and pests such as weeds and insects.
Potassium used in the manufacture of fertilizers comes from sedimentary salt beds left behind following the evaporation of ancient seas and lakes. Nearly all potassium fertilizer is in the form of potassium chloride. The potassium fertilizer industry started in Western Europe, where there are significant deposits of such ores. North America has the world’s largest reserves of potassium deposits. Other parts of the world containing potassium deposits include Brazil, China, Israel, Jordan, and Russia.
Sulfur (The Synthesizer)
Sulfur (S) is one of the most abundant elements in the soil and is one of the first elements scientists described. Like nitrogen, it is an essential component in the life of a cell. Sulfur is a component of the amino acids methionine and cysteine, which are used in the synthesis of proteins in all living things. Sulfur also is needed by enzymes associated with photosynthesis and chlorophyll synthesis. Sulfur is extracted from deep, naturally occurring underground deposits; from natural gas and crude oil; from the smelting of certain metal ores; and from gases produced by burning coal.
Calcium (The Supporter)
Calcium (Ca) plays a role in plants that is in some sense similar to that in humans. It is required for healthy growth and proper structural support. Calcium promotes proper cell elongation and is an essential part of the cell wall, helping to provide stability and bind cells together. In addition to its structural role, calcium participates in metabolic activities that help plants take up other nutrients and protect themselves against the effects of heat stress and infection by pathogens. Agricultural lime is used to supply plants with an external source of calcium. It is made from pulverized limestone or chalk, and the active ingredient is calcium carbonate.
Micronutrients
Among the micronutrients, the following four types of deficiencies are commonly addressed with fertilizers:
- Boron (B) is an essential nutrient in the growth and development of new cells. In plants, boron helps regulate flowering, pollination, seed development, and sugar transport.
- Copper (Cu) is a critical regulator of several plant enzyme systems and is necessary for protein synthesis and nitrogen metabolism.
- Manganese (Mn) is a part of several plant enzyme systems and plays a role in photosynthesis by regulating chlorophyll synthesis.
- Zinc (Zn) is an essential enzyme regulator involved with the synthesis of protein, starch, and growth hormones.
Organic and Commercial Fertilizers
During the past 30 years, the United States has witnessed a large growth in what is called organic farming. Although organic farming can be complicated to define, generally it relies on methods that use products that have been designated as “natural.” Today, the organic foods industry has annual sales of approximately $21.1 billion, with organic products representing 3 percent of all U.S. grocery spending.
Farmers who fertilize their crops have the choice of using either organic or commercial fertilizers, or a combination of the two types. As the name suggests, organic fertilizers come from once-living material, such as plants or animal manure. Commercial fertilizers consist of natural ingredients that have been subjected to a chemical process to make a fertilizer with increased and uniform nutrient content. Commercial fertilizers come either from natural mineral deposits or, in the case of nitrogen, from Earth’s atmosphere. Chemically, there is no difference between the nitrogen atoms that come from fertilizer, animal manure, a compost pile, or the atmosphere; provided they are in the same form (e.g., ammonium, nitrate, or urea), they are the same as far as the plants are concerned. However, there are differences in the rate at which the nitrogen from each of these sources is made available to plants and in the ratio of nitrogen to other elements such as phosphorus.
A major distinction between organic fertilizers and commercial ones is the quantities of nutrients they contain and the farmers’ knowledge about those quantities. Unlike commercial fertilizers, organic nutrient sources typically do not come with a guarantee of nutrient content. Organic material may supply high levels of one nutrient but low levels of another, creating an imbalance for crop plants. In contrast, commercial fertilizers contain known and often higher quantities of nutrients, which makes it convenient for farmers to apply them at rates that ensure that growing plants’ needs are met and nutrient losses to the environment are minimized.
An advantage of plant-based organic fertilizers (e.g., compost) is that the nutrients are released slowly so they are less likely to be supplied faster than plants can use them. For this reason, they are often considered less damaging to the environment than commercial fertilizers. However, manure-based organic sources are typically more volatile—or subject to movement—in the environment than commercial fertilizers. If any nutrient—commercial or organic—is applied at a rate higher than plants can use them, the excess may run off the fields or disperse into the air and contribute to nutrient pollution. Most organic fertilizers used on farms are manure based.
Meeting Crop's Nutrient Needs

Fertilizers can be applied as liquids or solids or, as in the case of anhydrous ammonia, as a pressurized gas that is injected into the soil. Bagged fertilizers are sold in a wide variety of mixtures. It is easy to read their contents from the fertilizer label. The percentage by weight of the three macronutrients—–nitrogen, phosphorus, and potassium—is listed as the fertilizer’s NPK ratio. For example, a label with an NPK ratio of 26-6-6 means that the fertilizer contains 26 percent nitrogen, 6 percent phosphorus, and 6 percent potassium. Some fertilizers also contain micronutrients, which are nutrients needed in smaller amounts for plant health and growth. Fertilizer labels also indicate the amounts of micronutrients as well as any inert ingredients such as sand that are included to provide bulk and make the fertilizer easier to apply.
Farmers use scientific methods to determine the appropriate nutrient balance for their crops. Because every farm field is different, farmers need to be able to select best-management practices (BMPs) that are suited to their growing conditions. Factors that may influence BMP selection include soil, climate, topography, and crop nutrient requirements. Often, farmers work with a certified crop adviser, a trained nutrient management professional, to assess growing and environmental conditions and develop a nutrient management plan.
The soil composition of farm fields varies greatly—often even on the same farm. Ensuring that crops get the precise amount of nutrients needed while minimizing nutrient losses to the environment involves consideration of a number of variables including the existing nutrient content of the soil and the crop nutrient needs. Soils can contain rich reserves of nutrients. Before fertilizing, farmers must measure the existing amounts of nitrogen, phosphorus, and potassium already in the soil. Then they can select a fertilizer that meets the needs of their crops. Sometimes, fertilizers are custom-blended to meet the farmer’s needs. After the right product has been selected, the nutrient management plan must also take into account the criteria described in Table 11. 
Organic or Commercial: Which is Better?
A quick answer to the question of which fertilizer is better is that neither organic nor commercial nutrient sources are better for plants. Both have their places and should be used where appropriate, and each has its advantages and disadvantages. Farmers need to examine the relative merits and decide when and where each type of fertilizer should be used. Because most organic fertilizers used on farms are from livestock, we focus here on manure-based organic fertilizers.
Manure-based organic materials encourage the use of local natural resources. They use little or no synthetic additives. Manure fertilizers may be viewed as economic and agronomic nutrient supplements along with commercial fertilizers in the production of crops. They contain varying amounts of plant nutrients and provide organic carbon, which is part of any productive agricultural soil. They improve the biological, chemical, and physical properties of soils. There are, however, some concerns associated with certain forms of manure-based organic fertilizer. First, when animal manures are produced in confined areas, excessive amounts of nutrients can accumulate in crop fields if the manure is over-applied near the site where it was produced. This can pose a threat of nitrate leaching to groundwater and phosphorus moving into surface waters through runoff and erosion. Second, the relatively fixed nutrient ratios of organic fertilizers can result in too much phosphorus being present in heavily manured soils because crops usually require much less phosphorus than nitrogen. In addition, significant amounts of ammonia gas (NH3) can be lost to the atmosphere.
By comparison, plant-based organic fertilizers are usually low in nutrient content. They contain some soluble nutrients, but most are released slowly as microbes in the soil break down the organic material into water-soluble forms that the plant roots can absorb. This feature may be an advantage when fertilizer is applied infrequently because it is less likely to overwhelm the system with soluble nutrients, which can result in nutrient loss to the environment. However, it also makes it difficult to time the release of nutrients to match the needs of the growing crop.
Commercial fertilizers contain precise, guaranteed levels of nutrients in forms that are readily available for plant uptake and use. It is possible to time their application to meet crop requirements, assuring efficient nutrient use and minimizing any potential impact on the environment. Because of their high nutrient content, commercial fertilizers are easy and economical to ship great distances from their point of production.
However, the high nutrient content of commercial fertilizers also means that the potential for overuse is greater. Farmers need to apply commercial fertilizers as specified by a nutrient management plan that is designed for the specific conditions of their fields. Nutrient management plans use data from soil and plant-tissue testing to help farmers use the proper amounts of nutrients at the optimal times. Nutrient management plans also keep farmers from wasting money by using too much fertilizer and from contributing unwanted nutrients to the air, groundwater, and local waterways. For example, although agriculture practices such as plowing can increase soil erosion, well-managed agricultural soils have less erosion than soils without an appropriate balance of nutrients. This is important because soil erosion in areas such as the Gulf of Mexico is an important contributor of nutrient pollution. 
Engage
- Ask your students to define the word, "symptom." A symptom is a sign or indicator of an illness or disease. As an example, ask students to recognize common symptoms of a cold or flu.
- Ask students if plants display symptoms when they are "sick" or lacking health. What type of symptoms would a plant show if it is not healthy? Allow students to offer their ideas and prior knowledge.
- Inform students that in this lesson they will:
- recognize that plants, like people, require essential nutrients to be present in the right amounts in order to be healthy,
- use reference materials to diagnose plant nutrient deficiencies,
- define fertilizer as a type of “food” for plants, and
- appreciate that fertilizers are used to replenish nutrients in agricultural soils.
Explore and Explain
Activity 1: When a Plant Needs “Food”
- Begin the activity by asking students how plants get the nutrients they need for growth .
- If necessary, remind students that plants obtain nutrients from the soil through the roots and transport them through the xylem tissue to the rest of the plant. Students may also mention photosynthesis. If necessary, remind students that in the case of photosynthesis, “food” refers both to the essential element carbon, which accounts for about half the weight of the plant, and to the light energy that is trapped and used to power plant metabolism. There are two important points that need to come out of this discussion. First, plants require essential elements (building blocks) that are not supplied by photosynthesis. Second, students should recall that essential elements are found in soil and absorbed by the plant through its root system.
- Remind students that plants and people are both made of cells and those cells need nutrients to be healthy. Ask, “What happens to us if we don’t get enough of an essential nutrient?”
- Student responses will vary. Students will recognize that when we have a nutrient deficiency, we are more likely to get sick.
- Continue the discussion by asking students to predict what might happen to plants if they do not get the nutrients they need. Ask students to work independently to write their ideas in their notebook or on paper. After students have written their own ideas, ask them to share their ideas with the class. Record answers on the board or chart paper.
- Keep this discussion moving and short. Accept all reasonable answers. Use probing questions to assess whether students think the plant’s response would be the same for all missing nutrients or whether there might be differences. Students will be investigating this question in the next activity and can revisit this list later.
Activity 2: Humanity Against Hunger WEB VERSION
Teacher note: Critical thinking is important to this activity. Not all of the information provided to students is helpful in identifying nutrient deficiencies. For example, the presence or absence of weeds in the fields is not a useful piece of information.
The Web version of this activity. One part of this activity, “Your Assignment for Humanity Against Hunger,” has an audio narration. If headphones are available, students can work at their own pace. In the event that neither headphones nor speakers are available for student computers, consider projecting the activity and showing the beginning of this part of the activity.
The web version allows students to type their initial observations into an online page that is identical to Master 4.3. However, the web program will not save this information. If you would like students to complete Master 4.3 as an assignment to turn in for a grade, you will need to provide them with a hardcopy of Master 4.3 and ask that they record their answers on it while conducting the simulation.
If the students’ computers are linked to a printer, then they can print an Award of Merit at the conclusion of the activity, which indicates that they completed all of the required steps correctly.
- For this activity, divide the class into groups of 3 students. Each group will evaluate 3 case studies. If sufficient computers are available, allow each student to work alone.
- Instruct the students to access the “Humanity Against Hunger” activity at https://www .nutrients forlife.org/games/humanity/.
- At the home page, instruct the students to begin by clicking on “The Food Crisis in Africa.” Ask volunteers to read parts of the article.
- This section serves as an introduction to agricultural issues in Africa and sets the stage for the next part of the activity during which students will investigate how nutrient deficiencies affect plant growth.
- Pause for a moment after reading for a brainstorming session with the students. Ask, “Can you think of ways to help solve Africa’s food shortage problem?”
- Instruct students to return to the home page and select “Your Assignment for Humanity Against Hunger.” At this point, the students will complete the Web activity on their own, following the directions given on the website.
- If you wish, project the first part of “Your Assignment for Humanity Against Hunger” up through the point where the narration gives instructions about what students will do during the activity.
- When students have completed the assignment, ensure that they print out their evaluation reports to turn in to you.
- After the assignment portion of the activity is completed, encourage students to explore the “Additional Resources/Links” section.
Activity 2: Humanity Against Hunger PRINT VERSION
This activity enables students to investigate how deficiencies in key nutrients result in observable changes in plant health.
- Project Master 4.1, Humanity Against Hunger and ask for a volunteer to read it aloud.
- Explain that students are going to review information sent in by local farmers who suspect that their crops suffer from a nutrient deficiency. Students will work to diagnose the specific nutrient deficiency affecting each crop plant. Students will refer to photographs and brief descriptions of four different nutrient deficiencies to help them make their diagnoses.
- Arrange the class into teams of 2–3 students. Pass out to each team 1 copy of Primary Information for each case study that they are to evaluate . Ask students to read Primary Information for their case studies.
- Each team receives the top portion of each page of Master 4.2a–c, Corn Case Studies. Each student in the team will be responsible for 1 of the case studies.
- Pass out to each team 1 cop y of Master 4.3, Plant Doctor Evaluation Form. Instruct students to write down in the appropriate space what they consider the important information related to their case study.
- Encourage students to discuss the information with their teammates and to work together to construct their answers.
- Pass out to each team 1 cop y of Master 4.4a–d, Plant Doctor Reference Manual. Instruct students to make a preliminary diagnosis for their case studies by using the information contained in the reference manual. Have students enter their initial diagnoses in the appropriate spaces on their evaluation forms.
- Remember, each student in the team is responsible for 1 of the 3 case studies. Students should list symptoms of the nutrient deficiency that matches the important information of their particular case study.
- Ask if teams are certain of their diagnoses.
- Some students may indicate that they have correctly diagnosed their case studies. Ask them what additional information would help them confirm or refute their diagnoses.
- Explain to the class that some additional information about their case studies has become known. Give each team the bottom portion of each page of Master 4.2a–c, Corn Case Studies, which contains Secondary Information.
- Ask teams to read the Secondary Information for their case studies and use this information to reevaluate their diagnoses. They should indicate on the evaluation form whether they want to confirm their initial diagnoses.
- If teams have changed the diagnosis, they should enter the new diagnosis together with the reason for the change in the appropriate spaces on the evaluation form.
- Ask students to use a different-color pen or pencil when they make changes or additions to their diagnosis or explanation. By doing this, both you and the students will be able to monitor changes in their thinking as they get more information.
- Reconvene the class and discuss each case study in turn, asking teams how they arrived at their diagnoses.
- Write the teams’ diagnoses on the board or chart paper.
- See Case Study Answers
- Project Master 4.5, Crops, Soil, and Nutrients, and pass out 1 copy to each student. Begin by asking volunteers to read aloud the information at the top of the page.
- Allow time for students to work in their teams of 3 to analyze the graph and answer the questions on the handout.
- Encourage students to discuss their ideas with their team members.
- Depending on your students, it may be helpful to look at the graph on Master 4.5 with the whole class before they begin answering the questions in their teams. The y-axis on the graph indicates the change in amount of a nutrient; it does not indicate the total amount of the nutrient in the soil. Zero on the graph indicates the starting amount of nutrients in the soil before crops have grown. This does not mean that the soil did not contain any nutrients. The direction from zero indicates whether there is more of a given nutrient in the soil after the crop has grown or whether there is less. For each nutrient and each crop represented on this graph, the level of nutrients in the soil is lower after the crops grow as indicated by bars that go down from the starting level.
- Hold a brief class discussion to review answers to the questions on Master 4.5 and to check their understanding.
- For this activity, the crop yields were based on an average yield for each crop grown in this country. However, yields will vary depending on a variety of factors including soil quality, water availability, and temperature, among others.
- Some students may have difficulty envisioning what a bushel is. If they are interested, you can tell them that a bushel of corn is approximately 56 pounds. A bushel of either soybeans or wheat is approximately 60 pounds.
- Teacher note: The data for the graph on Master 4.5 were obtained using the Nutrient Crop Tool on the USDA website (http://plants.usda.gov/npk/main). On this site, you can investigate nutrient needs for a wide variety of plants and you can adjust for number of acres and crop yield.
Optional Extension Activity: Investigating Calcium Deficiency
Preparation:
There are several ways of carrying out this optional extension activity. The procedure calls for students to plant pea seeds and then do the hands-on investigation. This will take class time over a couple of weeks. Another option is for you to plant the pea seeds and then, when the plants are a few days old, have the students take over to do the experimental treatment of the plants. Both of these options present good hands-on experiences for students and emphasize several practices of science. If class time is limited, you could have students analyze the photographs of the pea plants on Master 4.9, EDTA Effects on Pea Plants, rather than conducting the activity in a hands-on manner.
This activity uses ethylenediaminetetraacetic acid (EDTA) to mimic a calcium deficiency for plant growth. EDTA chelates divalent cations such as Ca++ thereby making calcium unavailable to the growing plants.
EDTA can be purchased from a scientific supply company. You should purchase ethylenediaminetetraacetic acid, disodium salt, dehydrate (C10H14N2Na2O8 • 2H2O) that has a molecular weight of 372.24. Because this chemical is used in molecular biology experiments, you may already have it in your supplies.
If you have 10 teams of students, 1 L each of 25 and 5 mM EDTA solution should be adequate. If you have more or fewer teams, you can adjust accordingly.
Prepare 1 L of at 25 mM EDTA solution as follows:
- Add approximately 800 mL of tap water to a large beaker.
- Add 1 N NaOH solution to raise the pH of the water to 8.
- Slowly add 9.3 g of EDTA to the water in increments, waiting until it dissolves before adding more.
- If the EDTA does not go into solution, check the pH and add more NaOH if necessary (pH needs to be basic).
- After all of the EDTA is in solution, check the pH again. The pH does not need to be exact but should be between 7 and 8.
- Add water so that the final volume of solution is 1,000 mL (1 L).
Prepare 1 L of 5 mM EDTA solution as follows :
- Pour 200 mL of 25 mm EDTA solution (prepared above) into a separate graduated cylinder.
- Add water to a final volume of 1 L (1,000 mL).
Procedures:
- Project Master 1.3, Essential Elements for Plants and point out that calcium is considered an essential nutrient for plants . Ask students to predict (in general terms) what they think might happen if a plant does not have its need for calcium met.
- Based on what they learned in Activity 2, students should suggest that there would be some physical manifestation of calcium deficiency that could be observed as a change in color, size, or overall health of the plant.
- Explain to students that they are going to investigate how calcium deficiencies affect plant growth. They are going to use a chemical called EDTA (ethylenediaminetetraacetic acid) to create a calcium-deficient environment for plants experimentally. Inform students that EDTA is a calcium chelator —in simple terms, EDTA binds the calcium in a way that makes it unavailable to the plant.
- Write on the board the question , “What effects does calcium deficiency have on the growth of pea plants ?” Inform stu dents that they will answer this question experimentally.
- Having this question visible where students can refer to it will help keep the testable question in students’ minds and help focus their attention.
- Give each student 1 copy of Master 4.6, Investigating Calcium Deficiencies. Also, project the first page of the master. Review the steps in the procedure with students and guide a discussion using questions such as the following to make sure they understand the experimental design.

- Ask students to work in teams of 3 for this activity. Allow time for teams to plant the pea seeds that will begin the experiment.
- Teams will need to make sure that the cups holding the seeds are watered periodically so they do not dry out. It will take approximately 5-7 days after planting until the seedlings are 5-7 cm tall and ready for the experimental treatments to begin.
- Once the treatments begin, teams should see results in about 1 week.
- If the pea plants start getting tall and falling over, you may want to use a skewer or stick to keep each plant upright and not falling down and getting tangled with other plants.
- If appropriate, encourage students to take photographs of the plants as an additional way to record data.
- At the conclusion of the experiment, ask students to record final observations on their data chart. Next, ask students to record their information on a class chart similar to the following. Provide a place where students can record observations to share with the class.
- After all teams have added their data to the class data chart, instruct them to calculate average values for each column.
- Conduct a class discussion that summarizes the effects of calcium depletion that were observed as part of the investigation.
- The data that they collected about plant height will likely reveal that plants watered with EDTA did not grow as tall as plants watered with tap water and that the plants watered with 25 mM EDTA were more severely affected than plants watered with 5 mM EDTA. In addition, students should have observations about the leaves or other parts of the plants that they can share.
- The following photographs and data show results of some of the experiments conducted during development of this curriculum. They can be used a representative data.

- The following photographs and data show results of some of the experiments conducted during development of this curriculum. They can be used a representative data.
- The second experiment seen below included treatment with 5 mM EDTA and liquid fertilizer (Miracle Gro mixed according to package directions). The fertilizer treatment is not included in the current procedure.

- The following data provide another way to look at the effects of EDTA, and liquid fertilizer in this experiment, on the growth of pea plants. If students collect data from their own experiments, they can compare this data with their own. Alternatively, you can share this data with them during a class discussion.
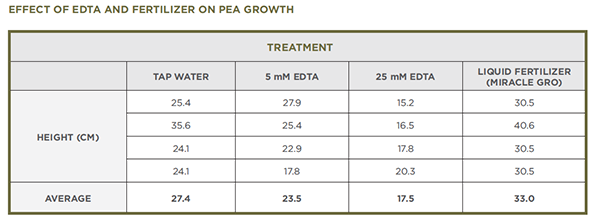
- The data that they collected about plant height will likely reveal that plants watered with EDTA did not grow as tall as plants watered with tap water and that the plants watered with 25 mM EDTA were more severely affected than plants watered with 5 mM EDTA. In addition, students should have observations about the leaves or other parts of the plants that they can share.
- Project Master 4.7, Additional Experimental Results. Explain that this handout shows photographs of plants that were watered with either tap water or 25 mM EDTA. Ask students to make observations from the photographs. Record observations on the board or chart paper.
- Students will notice that both plants looked healthy and were bushy and green before the experiment started. At the conclusion of the experiment, students should observe that the control plant (tap water) remained healthy, green, and bushy. The plant treated with 25 mM EDTA seems to have many dead leaves near the base, fewer leaves, and the remaining leaves are less green and there may be some dead or dying areas around the edge of the leaves.
- Pass out to each student 1 cop y of Master 4.8, Calcium and Plant Growth . Explain that this handout provides additional information about the role of calcium in plants and the effects of calcium depletion. Ask students to remember their experimental results as they read this new information.
- This reading provides a brief overview of some of the effects of calcium depletion. Many of the effects of calcium deficiency are observed on the most actively growing parts of the plant. The plant shown in Master 4.7 (after treatment with 25 mM EDTA) shows some of these traits. For example, you can see that there are many dead leaves around the base of the plant. These were new leaves that died before reaching maturity. In the older leaves, there were some spots and changes, but those took much longer to appear in the mature tissue compared with new tissues. Although not shown in the photographs, there were distinct changes in the root system of the EDTA-treated plant compared with the control plant. The roots were much less developed. In addition, when both plants were watered, the EDTA-treated plant did not take up as much water as the control plant.
Elaborate
-
Optional Homework Assignment: Instruct students to work with a parent/guardian for this activity. They will obtain a soil sample from near where they live. They can use the phone book or the web to find an address for the local county cooperative extension office or state university that conducts soil testing. Students should send in their soil sample for analysis to assess its quality and to see if any essential nutrients are lacking. You can collect the soil analyses obtained by different students and see if there are any differences according to location. Note that some states and universities will assess a fee for soil testing, which is typically no more than $10. Private organizations will charge more. Factoring in the time for the organization to conduct the test and prepare a report, this assignment is better used as a long-term project or science fair project.
-
This lesson is the fourth in a series of five related lessons. Refer to the following lessons for further depth.
- Lesson 1: In Search of Essential Nutrients
- Lesson 2: Properties of Soils
- Lesson 3: Plant-Soil Interactions
- Lesson 4: Plant Nutrient Deficiencies
- Lesson 5: Fertilizers and the Environment
Evaluate
After conducting these activities, review and summarize the following key concepts:
- The growth of plants in nature and the growth and management of plants by farmers for the purpose of food, fiber, and fuel are different.
- Examples of organic fertilizers include compost and animal manure. Examples of inorganic fertilizers include various formulations of commercial fertilizer which are designed to return nutrients to the soil.
- Sustainable agriculture includes using techniques that protect the environment and preserve natural resources for future use.
- Natural cycles govern the flow of nutrients in the environment.
Sources
- Nutrients for Life Foundation
- BSCS-Biological Science Curriculum Study
- Reviewed by Smithsonian Institution
Recommended Companion Resources
- Nutrients for Life eLessons
- Phosphate Mining Video
- Potash Mining Video
- Soil, Not Dirt
- Feeding the World and Protecting the Environment
- Mobile System Removes Phosphorus From Manure
- Smartphones Enlisted to Battle Crop Disease
- All About Corn: e-learning modules for educators and students
- Indoor Gardening Curriculum
- Nitrogen & Agriculture
- Soil Health Education Resources
- Web Soil Survey
Author
Nutrients for Life Foundation
Organization
Nutrients for Life Foundation
| We welcome your feedback! If you have a question about this lesson or would like to report a broken link, please send us an email at matrixelearning@gmail.com. If you have used this lesson and are willing to share your experience, we will provide you with a coupon code for 10% off your next purchase at AgClassroomStore. |
