 Relevancy and Engagement
agclassroom.org
Relevancy and Engagement
agclassroom.org
Biofuels and Bioproducts (Grades 6-8)
Grade Level
6 - 8
Purpose
Students explore the process of fermentation in the creation of ethanol and observe the role enzymes play in the fermentation of starch. Grades 6-8
Estimated Time
2 hours
Materials Needed
Activity 1:
- Fermentation Factories student handout, 1 copy per student
- Yeast
- Warm water (95° F/35° C)
- Liquid glucose or crushed glucose tablets
- Corn flour
- Amylase
- Glucoamylase
- Snack-sized bags
- Possible materials for student use:
- Snack-sized bags
- 50 ml water
- 1 tsp. yeast
- ¼ tsp. enzymes (amylase, glucoamylase)
- 1 tsp. sugars (simple & complex) as feedstocks: corn flour, corn starch, corn syrup, honey,
and glucose - Ruler to measure gas volume
- Index card or clipboard to measure gas volume
Activity 2:
- Ticketase student handout, 1 copy per student
- 3 strings of 50 connected tickets per student group to represent the corn flour polysaccharide molecule
- Timer (optional)
- Blindfold (optional)
Vocabulary
biofuel: a fuel derived directly from living matter
dent corn: a variety of grain corn high in starch content; named for the dent at the crown of each kernel
distillers grain: a cereal byproduct of the distillation process
enzyme: protein catalyst, which speeds up a specific chemical reaction
ethanol: a fuel produced by fermentation of products high in starch, such as corn
nonrenewable fuels: fuel produced from a source that cannot be readily replaced at the rate it is consumed
renewable fuels: fuels produced from renewable resources such as biofuels and hydrogen fuel
Did You Know?
- Corn is the primary grain grown for livestock feed in the United States.1
- More than 90 million acres of land are planted to corn in the United States.1
- Corn is grown for food, livestock feed, ethanol fuel, and other industrial uses.1
Background Agricultural Connections
 Human consumption of fuel is on the rise as both population and affluence steadily increase. Renewable fuels, such as ethanol, can help to decrease the need for non-renewable fuel sources such as crude oil. In addition, ethanol has replaced methyl tertiary-butyl ether (MTBE) as the major octane source in gasoline which has resulted in gasohol blends (ethanol plus gasoline) of up to 10% in almost every pump in the United States. Ethanol is a renewable fuel source that is both energy positive, which means it generates more energy than it takes to make it, and helps to reduce greenhouse gas emissions. Here is the equation for the fermentation of glucose into ethanol and carbon dioxide:
Human consumption of fuel is on the rise as both population and affluence steadily increase. Renewable fuels, such as ethanol, can help to decrease the need for non-renewable fuel sources such as crude oil. In addition, ethanol has replaced methyl tertiary-butyl ether (MTBE) as the major octane source in gasoline which has resulted in gasohol blends (ethanol plus gasoline) of up to 10% in almost every pump in the United States. Ethanol is a renewable fuel source that is both energy positive, which means it generates more energy than it takes to make it, and helps to reduce greenhouse gas emissions. Here is the equation for the fermentation of glucose into ethanol and carbon dioxide:
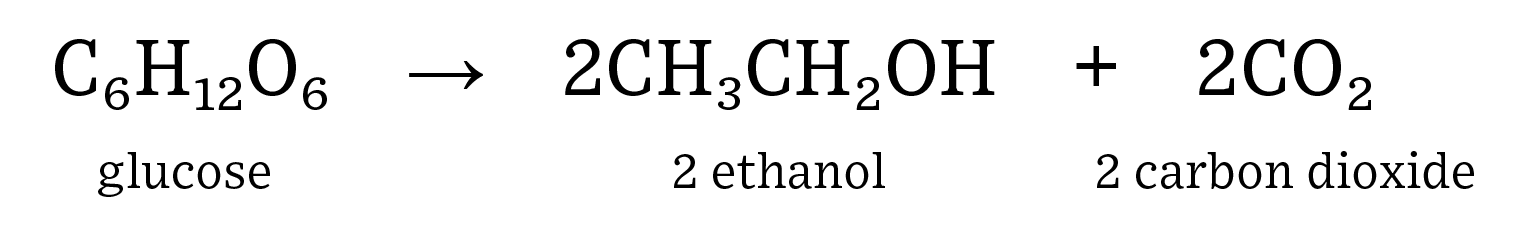 Students should be familiar with the concept of respiration: release of energy requiring the use of oxygen, and fermentation: an anaerobic process that releases energy. Yeast are used in this model to show fermentation. Ethanol is created when yeast consume glucose (simple sugar). Ethanol in the United States is produced by breaking down corn flour to create glucose, which is then consumed by yeast to produce CO2, ethanol, and distillers grains. Distillers grains are the leftover corn fiber, protein, and oil that result from the breakdown of starch in corn.
Students should be familiar with the concept of respiration: release of energy requiring the use of oxygen, and fermentation: an anaerobic process that releases energy. Yeast are used in this model to show fermentation. Ethanol is created when yeast consume glucose (simple sugar). Ethanol in the United States is produced by breaking down corn flour to create glucose, which is then consumed by yeast to produce CO2, ethanol, and distillers grains. Distillers grains are the leftover corn fiber, protein, and oil that result from the breakdown of starch in corn.
Engage
- Show an image of a busy, multilane highway to the students.
- After showing the image, ask students to brainstorm questions individually for 30 seconds to one minute, then share their questions within small groups (3–4 students) for the next two or three minutes.
- Have groups share their questions one-by-one to the large group until all questions are shared.
- Create a Driving Question Board to keep note of the questions, as they will guide the rest of this unit.
- Teaching Tip: For more information on driving question boards, see The Science Teacher (November 2008), Vol 75, No 8.
- Possible questions:
- How many vehicles does the average family in the United States own?
- How many vehicles are in use worldwide?
- Why do we need so many vehicles?
- How many vehicles are in use on average per day?
- How much fuel is consumed on average per day?
- What forms of fuel are available?
- What impact can these fuel types have on the environment?
- Can we extract enough petroleum to meet this growing demand?
- Can we create enough ethanol to create gasohol blends?
- How will human growth impact fuel consumption?
- If no one brings up fuel consumption or fuel production, add your own questions:
- Do we have enough fuel to power these vehicles?
- What types of fuel are available for these vehicles to use?
- What methods are used to produce these fuel types? How will human growth impact vehicle use and fuel consumption in the future?
- To begin investigating these questions, organize the ones that relate to the sheer number of vehicles and the rate at which vehicle use has grown.

Explore and Explain
Activity 1: Fermentation Factories
How can we create ethanol? What does the process of fermentation produce?
Preparation: Create the following bags 25–30 minutes prior to class. If possible use warm water (95° F/35° C) to hydrate the fermentation bags. Remove all of the air from the bags, seal, and incubate the bags in a warm location (98.6° F/37° C) for optimum fermentation. Remove the bags from the incubator and ask the students what they are observing. Allow the students to generate discussion with their observations. Do not confirm or deny ideas as you lead the conversation with your students.
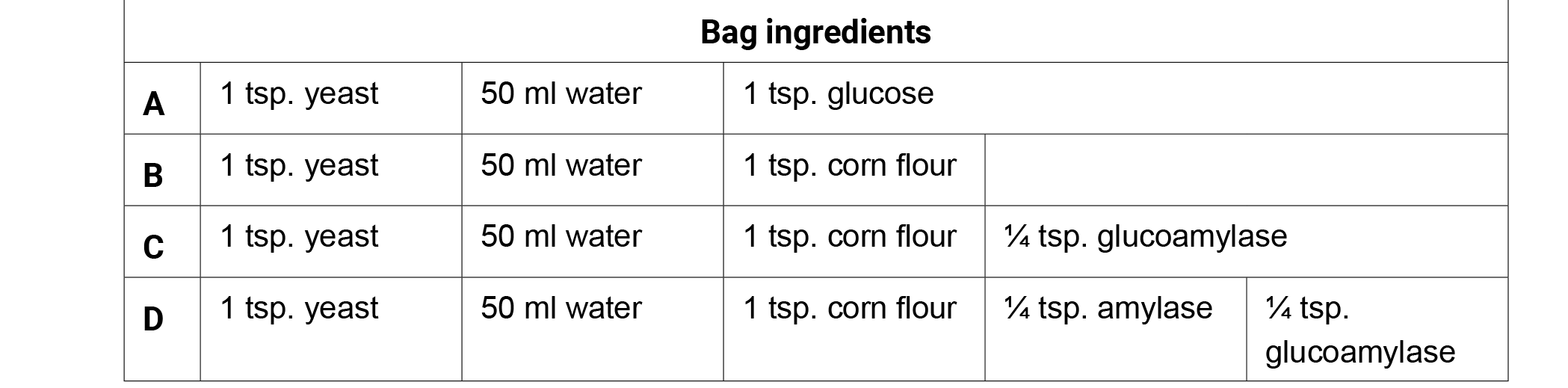
- Give each student one copy of the Fermentation Factories student handout.
- Write the ingredients of each bag on the board and have students brainstorm observations or questions surrounding the function of each ingredient individually for 1 minute. Have them record both the bag contents and their observations on their charts for later use.
- Next, have the students share their observations in a small group for three minutes. Generate class discussion by asking groups to share their observations with the class. Possible observations or questions about the corn fermentation in a bag ingredients:
- Glucose is a simple sugar (monosaccharide).
- Yeast are organisms/decomposers that eat sugars.
- Starch is a complex sugar (polysaccharide).
- Fermentation occurs when yeast consume sugar (glucose) and produce alcohol (ethanol) and carbon dioxide.
- Bag A produced the most CO2 in 20 minutes (glucose).
- Bags B and C produced very little CO2 in 20 minutes.
- Bag D produced the second largest amount of CO2 in 20 minutes.
- What do amylase and glucoamylase do? How do they function with sugars or yeast?
- Read the challenge to the students: Create the greatest volume of ethanol (measured by the volume of CO2 generated) in the fastest time possible.
- Students should work in groups of 2–3 individuals for this challenge. Review the following criteria and constraints for the challenge.
- Plan an (several) experiment(s) to produce ethanol in a small bag environment.
- You can only use the following materials/amounts provided.
- You have 1 or more class period(s) to experiment on your initial design(s) based on your plan.
- Data must be collected and analyzed to provide evidence for your conclusion.
- Report back to the class and provide future experimental designs as a result of your current data/conclusion.
- Provide some, all, or additional material items to the students that are listed above.
- Discuss the engineering design process with your students. Encourage student groups to create two or more experimental designs based upon their knowledge of what occurs in the phenomena bags. Why are they investigating their design? What is their reasoning for their materials? What patterns do they expect to see? They will also need to create a method for measuring their CO2 gas. We suggest that they measure volume by height displacement using a clipboard and ruler to demonstrate their volume change in CO2 gas.
- Students should be able to predict the outcome of some of their experimental designs based upon previous background knowledge and their observations of the anchoring phenomena.
- Encourage the students to create charts and graphs to show the volume change within their bags over time. Students should create their own experimental procedure to collect and record data.
- Students should create a model of their fermentation ecosystem. The students can draw what is happening in the anaerobic process of fermentation based on the data collected in their experimental models.
- Create a discussion with your students to determine if they could improve upon their experimental design based upon the evidence presented. What could they improve upon? Materials used? Experimental conditions? What research could they do to make their design as efficient as possible?
- Can you create a more efficient design using different materials?
- Can you predict the outcome of other experimental designs?
- How can you change you original design to become more efficient by changing the experimental conditions?
- Make predictions using all of the available feedstocks in separate designs to determine which one will make the most CO2 over time.
Activity 2: Ticketase
What roles do different enzymes play in the fermentation of starch?
- Give each student one copy of the Ticketase student handout.
- Remind the students of the 4 fermentation bags used in Activity 1. What was occurring in each of the four bags? You can help guide the students’ discussion by asking questions as you record their observations/questions.
- How did the amylase and/or glucoamylase impact the fermentation reaction?
- What are the roles of amylase and glucoamylase?
- Did amylase/glucoamylase help the fermentation process occur more slowly, rapidly, or have no net effect?
- Have students work together in groups of 4. Provide each group with 3 strings of 50 connected tickets. Instructions for the activity are included on the student page.
- Refer to the Ticketase Teacher Key for answers to the handout, ideas for differentiation, and a rubric for assessment.
Elaborate
Explore graphs and charts from the National Corn Growers Association to see where corn is grown, corn yield statistics, what corn is used for, and more.
Evaluate
After conducting these activities, review and summarize the following key concepts:
- Ethanol is created using the process of fermentation.
- Enzymes break molecules like starch into smaller molecules that can be used by yeast for the fermentation of sugars.
Sources
Acknowledgements
This lesson is an excerpt of the Biofuels and Bioproducts unit created by Nourish the Future.
Recommended Companion Resources
Author
Nourish the Future
Organization
Nourish the Future